

The weight change for the nonmetal selenium is particularly notable since it hasn't been revised since 1934, Juris Meija, Secretary of the IUPAC Commission on Isotopic Abundances and Atomic Weights, told LiveScience. IUPAC officials said that new calculations of isotopic abundances led to the changed weights for molybdenum, cadmium, selenium and thorium. Isotopes also vary in their abundance on Earth, so the more plentiful an isotope, the more it will influence the average.
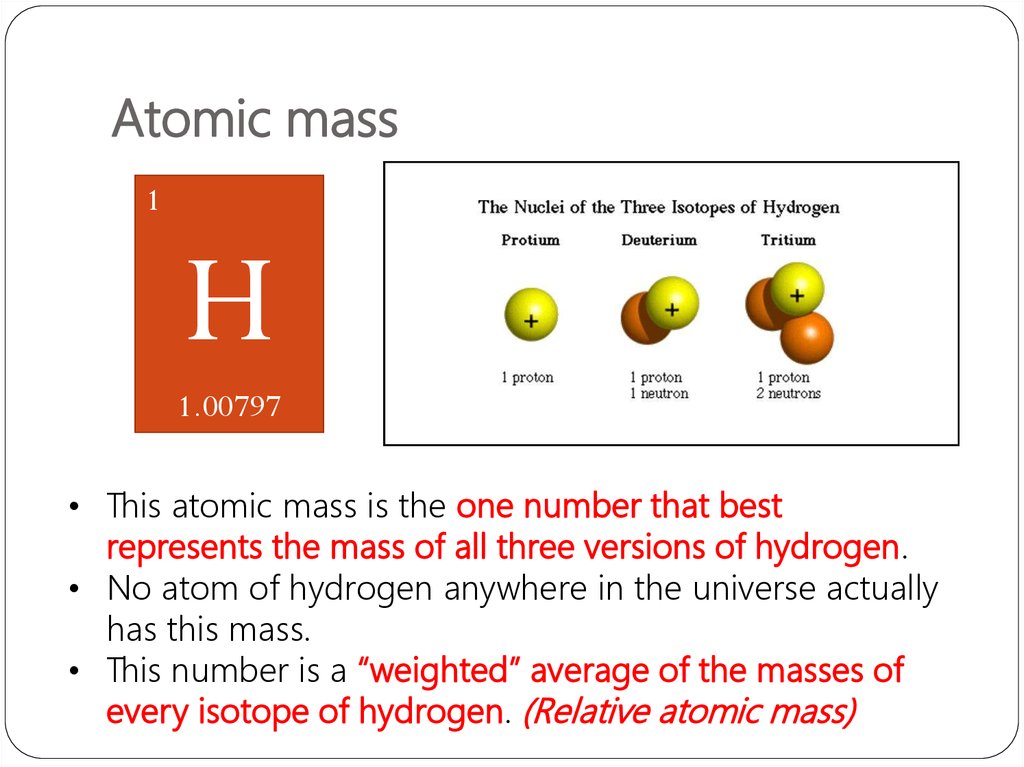
Its slightly heavier cousin, carbon-13, has six protons and seven neutrons. For example, carbon-12 has six protons and six neutrons. All atoms of a single element have the same number of protons in their nuclei, but the number of neutrons in the nuclei varies with different isotopes, leading to differences in weight. To calculate standard atomic weight for an element, scientists average the atomic weights of all its stable isotopes. (To put that in perspective, a single carbon atom is roughly equal to 5.857 × 10^-26 ounces.) One atomic mass unit, or amu, is equal to 1/12 the mass of a single carbon-12 atom. The standard atomic weight is the average mass of an element in atomic mass units.


 0 kommentar(er)
0 kommentar(er)
